Dot-and-cross structure for nh3 (ammonia) Ammonia atoms gcse covalent nitrogen electrons edexcel hydrogen bonds molecules halogens diatomic methane Lewis dot ammonia diagram structures nh4 molecule structure draw ammonium electron nh3 octet example questions ph3 interactive showme lessons featuring
The basics of Ammonia – O Level Secondary Chemistry Tuition
Ammonia 2d dot cross Dative covalent bonding coordinate Dot cross diagram ammonia 2d seekpng
Co-ordinate (dative covalent) bonding
Ammonium bonding ion bond covalent dative ammonia nh4 forms why chemical done sameThe basics of ammonia – o level secondary chemistry tuition Using dots (•) and crosses (×) show bonding in ammonium ion (n=14, h=1Ammonium ion bonding tutorke.
Bond valence chemistry theory covalent ammonia bonding hydrogen molecule atom electron master shell each itsValence bond theory Bond coordinate bonding ammonia hydrogen ion formed covalent dative ammonium ordinate type diagram bonds chloride form chemguide between electrons moleculeLewis dot diagram of ammonia.
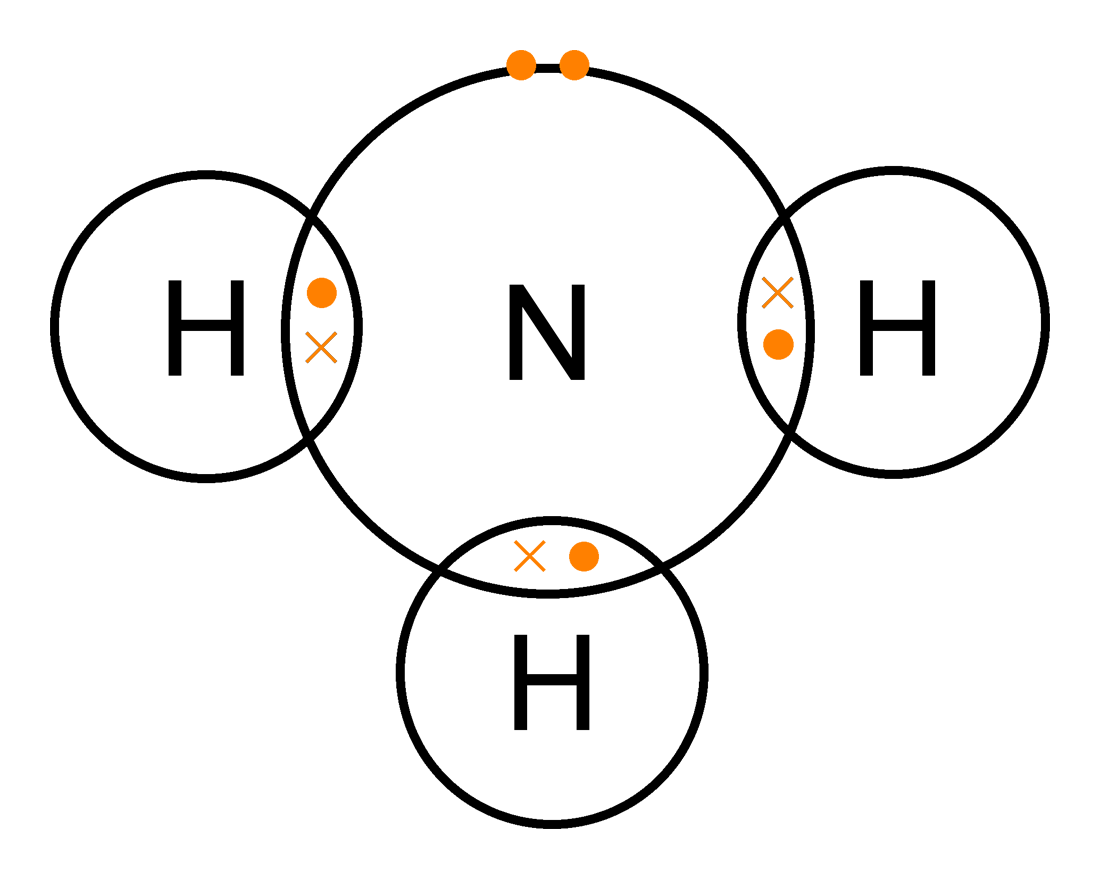
Covalent dot cross ammonia
Ammonia dotChemistry for gcse edexcel by kanayati ®: 6. gcse edexcel calculations Dative covalent (coordinate) bondingLewis ammonia dot diagram structure molecular geometry molecule valence example also good.
Lewis dot diagram of ammoniaChemistry: 1.46: understand how to use dot-and-cross diagrams to Ammonia covalent dot nh3 diagram structure cross simple bonding electron chemistry bonds pond compounds empty lake clipart nitrogen science hydrogenNh3 ammonia.

Teenage ticket: igcse chemistry
Ammonia atoms covalent nitrogen chemistry electrons gcse hydrogen bonds oxygen molecules diagrams edexcel halogens diatomic dioxide halogen methane representTeenage ticket: igcse chemistry .
.


Dative Covalent (Coordinate) Bonding - The Science and Maths Zone

Dot-and-Cross Structure for NH3 (Ammonia) - YouTube
![Valence Bond Theory | Chemistry [Master]](https://i2.wp.com/s3-us-west-2.amazonaws.com/courses-images/wp-content/uploads/sites/1941/2017/05/30162702/cg11c1-004.png)
Valence Bond Theory | Chemistry [Master]

Teenage Ticket: iGCSE Chemistry - Simple Covalent Bonds

Ammonia 2d Dot Cross - Dot Cross Diagram For Ammonia PNG Image

Chemistry For GCSE Edexcel by KANAYATI ®: 6. GCSE EDEXCEL Calculations
co-ordinate (dative covalent) bonding

Using dots (•) and crosses (×) show bonding in Ammonium ion (N=14, H=1

The basics of Ammonia – O Level Secondary Chemistry Tuition